Activ Surgical Completes First In-Human Procedures to Demonstrate Impact of its Surgical Intelligence and Sensing Platform
Activ Surgical, a digital surgery company, today announced the completion of its first in-human surgery clinical trial utilizing the company’s technology-as-a-service bundle—the ActivInsights augment reality (AR)-based software suite and the ActivSight imaging module. Procedures for the study at The University of Texas Health Science Center at Houston (UTHealth Houston) were led by Dr. Erik Wilson, professor in the Department of Surgery at McGovern Medical School at UTHealth and a leading bariatric, endoluminal and robotic general surgeon; and Dr. Shinil Shah, associate professor at UTHealth Houston and a preeminent general surgeon with expertise in advanced gastrointestinal surgery. The safety and feasibility study was conducted across bariatric, colorectal and gallbladder procedures—surgeries in which intraoperative assessment of perfusion and anatomic structures are critical to prevent costly and life-threatening complications.
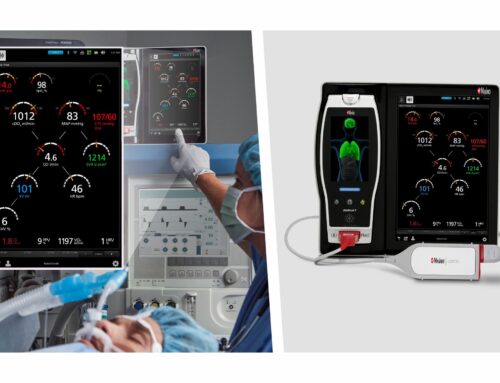
During the study, Dr. Wilson and Dr. Shah conducted a number of procedures using the ActivInsights software suite along with the company’s interoperable imaging module, ActivSight. Together, ActivInsights and ActivSight delivered Perfusion View Insights, which allowed Dr. Wilson and Dr. Shah the ability to see blood flow and perfusion in real time, without the use of traditional dyes.
“Patient safety is always our number one priority in the operating room,” said Dr. Wilson. “Our study using the ActivInsights AR-based software suite and the ActivSight imaging module revealed no safety issues and showed it has the potential to improve patient outcomes and reduce surgical complications. It was seamless for our team to use and provided physiological information in real-time during these first in-human procedures.”
The UTHealth Houston study’s procedures were performed at Memorial Hermann-Texas Medical Center and Memorial Hermann Sugar Land Hospital. The study with UTHealth Houston is the first of several in-human trials Activ Surgical has planned around the world with leading healthcare systems and renowned surgeons in 2021. The company received Food and Drug Administration (FDA) clearance for the ActivSight interoperable imaging module in April and expects its software suite to be commercially available to surgeons around the world in 2022.
“We are grateful to Dr. Wilson and Dr. Shah to be the first to participate in these imperative in-human safety and feasibility studies,” said Todd Usen, chief executive officer, Activ Surgical. “While HD and 4K have taken what a physician or a television viewer can see, and enhances it, the ActivInsights software suite and ActivSight imaging module takes this advancement a step further by allowing surgeons to see what they currently cannot via present-day technology. These trials, coupled with our recent clearance from the FDA, are critical steps in the rollout of our technology that will improve patient outcomes and safety in the OR and help to democratize surgical care across the globe.”
For more information about Activ Surgical and the ActivInsights AR-based software suite, please visit www.activsurgical.com.