Sanguina, Inc. has received FDA clearance for AnemoCheck Home, marking it as the sole FDA-cleared home hemoglobin test kit in the U.S. Designed for those with anemia due to nutritional deficiencies, sickle cell disease, and thalassemia, AnemoCheck Home aims to make it easier for users to keep an eye on their hemoglobin levels without leaving their residence.
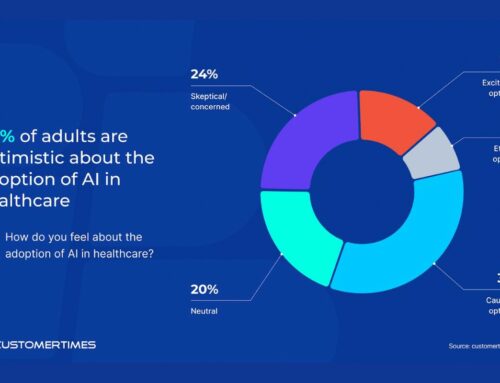
Anemia, characterized by a deficiency in red blood cells or hemoglobin, is a global issue affecting nearly 1.92 billion people. AnemoCheck Home has been introduced to the market as a response to the worldwide demand for accessible and dependable anemia testing.
The user-friendly procedure of AnemoCheck Home involves a straightforward fingerstick blood test. After collecting blood into a tube, the user connects the test cap to the test body and shakes it to mix the blood. A color change after 2 minutes corresponds to a specific hemoglobin level, as indicated on an accompanying color card. The entire kit is disposable and doesn’t necessitate any other equipment.
Sanguina’s CEO, Erika Tyburski, expressed her enthusiasm about the test’s potential impact, stating, “We’re proud to offer AnemoCheck Home, a pivotal tool for home-based anemia monitoring. Through this FDA clearance, we hope to give those with anemia a dependable and straightforward method to keep track of their hemoglobin levels.”
Embracing recent innovations in medical technology, AnemoCheck Home ensures accuracy in its results, giving users confidence in managing their anemia. The package comes complete with easy-to-follow instructions and all the essentials for the fingerstick procedure.
For more detailed information on AnemoCheck Home, readers can visit https://sanguina.com/anemocheckhome.