Novel Algorithm Leverages Artificial Intelligence (AI) to Identify Concussion Without the Need for a Baseline Test
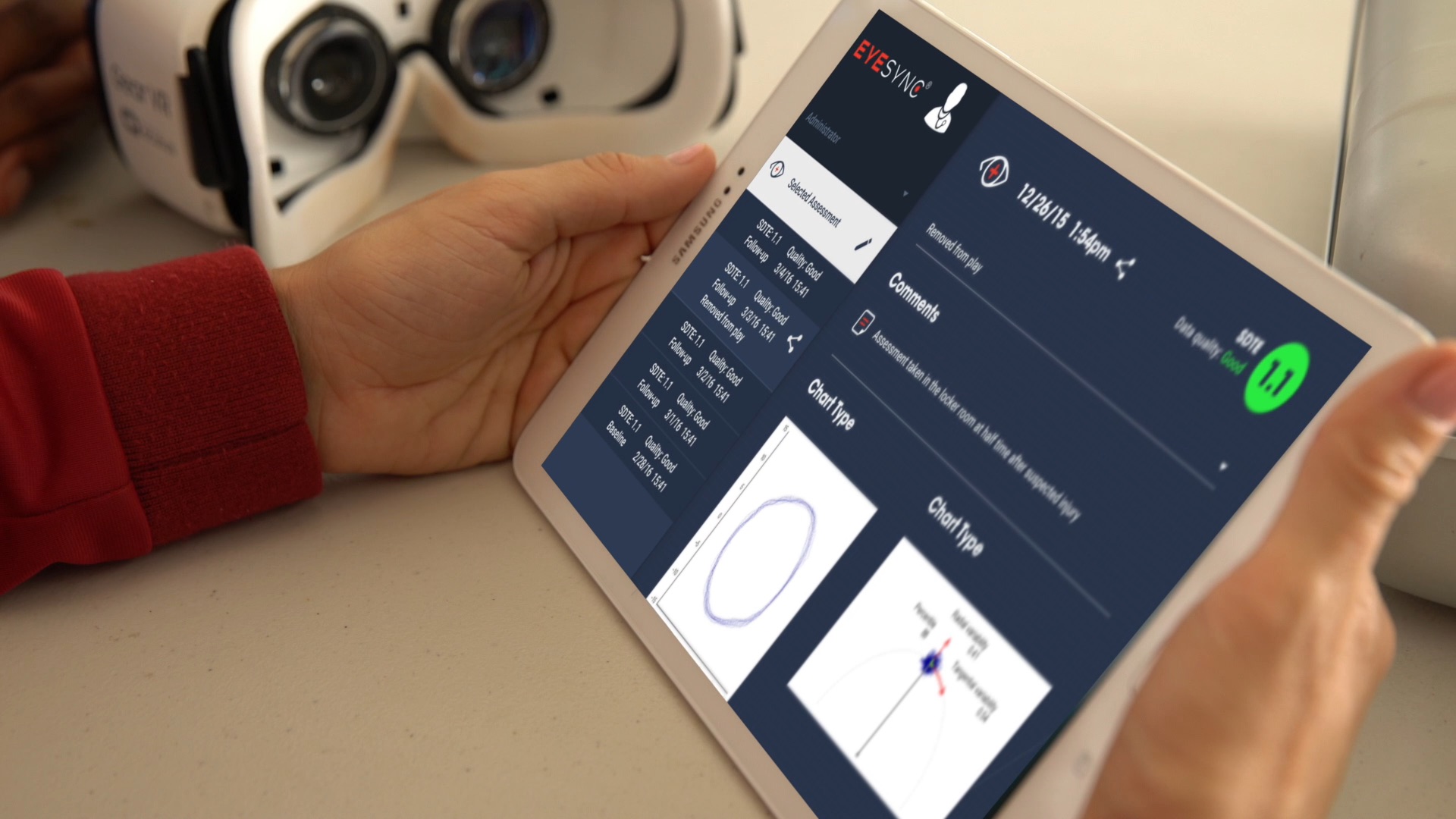 SyncThink, a neurotechnology company and developer of the award-winning EYE-SYNC technology, announces today the US Food and Drug Administration (FDA) has granted clearance of the EYE-SYNC technology as an Aid to Concussion, or mild Traumatic Brain Injury (mTBI) Diagnosis. EYE-SYNC previously received the prestigious Breakthrough Device Designation from the FDA in 2019, following the initial clearance and commercialization of the technology platform in 2017.
SyncThink, a neurotechnology company and developer of the award-winning EYE-SYNC technology, announces today the US Food and Drug Administration (FDA) has granted clearance of the EYE-SYNC technology as an Aid to Concussion, or mild Traumatic Brain Injury (mTBI) Diagnosis. EYE-SYNC previously received the prestigious Breakthrough Device Designation from the FDA in 2019, following the initial clearance and commercialization of the technology platform in 2017.
“After a decade of clinical research, 30 publications, and 16 patents, we are pleased to formally extend EYE-SYNC’s indications to the many providers who will benefit from rapid, objective diagnostic technology,” said SyncThink founder and Stanford University Professor Jam Ghajar, MD, PhD. “Concussion is a complex condition and a challenge for many clinicians to manage, so we hope that by offering highly accurate diagnostic tools and proven algorithms, we will help providers and their patients identify the most effective and appropriate treatment.”
In support of the application, SyncThink enrolled 1,655 pediatric and adult subjects into a clinical study that collected comprehensive patient and concussion related data for over one year. Utilizing this information, SyncThink implemented proprietary algorithms and deep learning models to identify a positive or negative indication of concussion. The study showed that EYE-SYNC demonstrated sensitivity above 82% and specificity above 93%, thereby providing clinicians with significant and actionable data when evaluating individuals with concussion.
“The outcome of this study very clearly shows the effectiveness of our technology at detecting concussion, and definitively demonstrates the clinical utility of EYE-SYNC. It also shows that the future of concussion diagnosis is no longer purely symptom based, but that of a technology driven multi-modal approach,” said SyncThink Chief Clinical Officer Scott Anderson.
The EYE-SYNC technology utilizes a series of 60 second eye tracking assessments, neurocognitive batteries, symptom inventories, and standardized patient inventories to identify the type and severity of dysfunction after concussion. The platform generates customizable and interpretive reports that support clinical decision making, and offers visual and vestibular therapies to remedy deficits and monitor improvement over time.
To learn more about EYE-SYNC, visit syncthink.com.
About SyncThink:
SyncThink is on a mission to provide objective neurological measurements that assists medical professionals in assessing brain health, delivering therapy, and optimizing performance. SyncThink develops revolutionary eye-tracking software and analytic technologies, delivered in a rapid, accurate, and easy to use medical XR platform. With more than 30 published peer-reviewed papers and 16 granted patents, the SyncThink platform uses a series of 60-second assessments to objectively measure eye movements to identify impairments and offers multiple modalities to train dynamic vision. FDA-cleared for detecting eye-tracking impairment as an aid to concussion diagnosis, and for detection of visual impairment and its use in related clinical conditions. The technology is used by over 3,500 users across leading medical institutions such as Stanford and the Massachusetts General Hospital, the U.S. military, and more than 20 universities. SyncThink is transforming neurological assessment, therapies, and performance for health and sport. www.syncthink.com