Senzime today announces that the company’s device, the TetraGraph has been compared with the TOFscan in a 120-patient, multicenter international study from the Mayo Clinic Jacksonville, USA; the University of Debrecen, Hungary; and the NorthShore University Health System, Chicago, USA. The study has been published ahead of print in the Journal of Clinical Anesthesia, Vol 71 (2021).
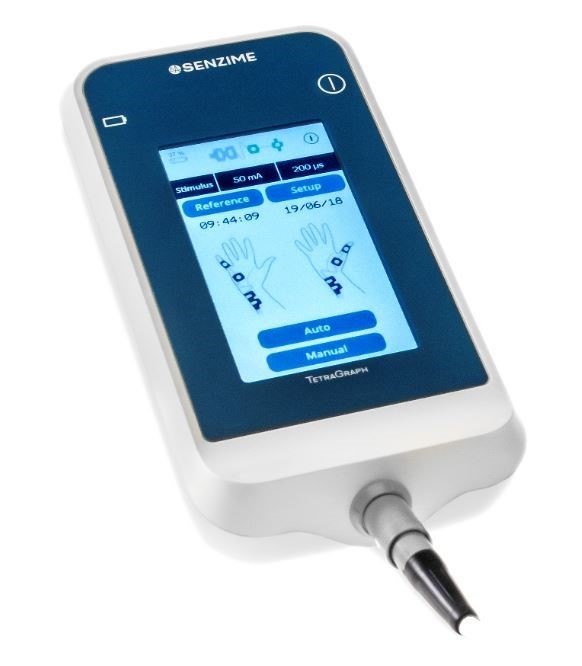
As stated in the article, “Comparison of the TetraGraph and the TOFscan for monitoring recovery from neuromuscular blockade in the Post Anesthesia Care Unit”[1], the lead author, Dr. J. Ross Renew reports that the number of patients arriving to the post-anesthesia care unit (PACU) with evidence of residual neuromuscular block (RNMB) remains alarmingly high despite the significant threat to patient safety that residual weakness poses. Quantitative neuromuscular monitoring is currently the only method to accurately and consistently exclude residual weakness, and experts as well as international anesthesia specialty organizations advocate for its use whenever NMBAs are administered.The article also points out that the use of sugammadex without quantitative monitoring does not eliminate the occurrence of residual weakness. The authors conclude that time constraints, limited access to devices, and gaps in knowledge have been barriers to quick adoption of quantitative monitoring.
The study compared the performance of the TetraGraph (electromyography, EMG) and the TOFscan (acceleromyography, AMG) in the PACU where complications from residual weakness often surface. The primary outcome was assessment of the agreement between TOF ratios obtained by the AMG and EMG monitors, while the secondary outcome was to determine the overall incidence of postoperative residual weakness and identify risk factors.
There was a significant difference between the three clinical sites with regard to the type of device used to assess recovery from neuromuscular blockade, as well as a significant difference in the type of pharmacologic antagonism used to restore neuromuscular function.
| Site | Monitoring technique | Pharmacologic antagonism |
| 1 | PNS (peripheral nerve stimulator) and subjective evaluation |
Higher portion of neostigmine (than site 2 and 3) |
| 2 | Various quantitative neuromuscular monitors | No use of antagonism in 26% of patients |
| 3 | A quantitative monitor a majority of time | Exclusively using sugammadex |
Among all 3 sites, a total of 13.9% of patients had residual weakness (RNMB). Not surprisingly, the use of a PNS was associated with 75% more postoperative residual weakness.
The TOF ratios between TOFscan and TetraGraph devices did not differ significantly upon arrival and 5 min after arrival, but they differed significantly 10 min later, which may be a result of involuntary withdrawal movements by awakening patients, which affect AMG-based measurements but do not interfere with EMG-obtained compound muscle action potentials. AMG has several other limitations. Baseline TOF ratios often exceed 1.0 and can lead to clinicians overestimating the degree of recovery when using AMG. Alternatively, EMG offers several advantages. Unlike AMG, EMG does not rely upon muscle movement and can therefor provide objective data when the arms are tucked under surgical drapes, they are not prone to measurement error due to involuntary muscle contractions, and the EMG-derived measurements remain consistent over time with a high level of agreement with mechanomyography (MMG).
“Standalone, handheld monitors are invaluable in diagnosing post-operative residual weakness in PACU patients. This chronic patient safety threat should be addressed with a multi-faceted approach that incorporates appropriate pharmacologic NMB antagonism with objective monitoring” says Dr. J. Ross Renew, PI, Dept of Anesthesiology and Perioperative Medicine, Mayo Clinic, Jacksonville, FL, USA
“Reading the outcome of this investigator-initiated study makes both me and my team incredibly honored to play a part in advancing patient safety in our race to zero complications”, says Pia Renaudin, CEO of Senzime.
About Senzime
Senzime develops and markets CE- and FDA cleared patient monitoring systems driven by unique algorithms and sensors to closely monitor patients under anesthesia. TetraGraph is a system that digitally and continuously measures the degree of neuromuscular blockade in the patient. The goal is improved clinical precision and simplified management in healthcare. By preventing complications and enabling healthcare professionals to follow guidelines and drug recommendations, TetraGraph can contribute to shorten hospital stays and lower healthcare costs. The vision is a world without anesthesia related complications, where everyone wakes up safely after surgery. Senzime operates in growing markets that in Europe and the United States are valued in excess of SEK 15 billion. The company’s shares are listed on Nasdaq
First North Growth Market (ticker SEZI). FNCA Sweden AB, +46 (0)8-528 00 399, info@fnca.se is Certified Adviser for Senzime. www.senzime.com