New Study Tracks Changes in Breast Cancer Therapies During the Pandemic
COVID-19 rapidly altered breast cancer treatment approaches with a significant rise in neoadjuvant endocrine therapy (NET) for estrogen receptor positive (ER+) tumors, enabling immediate evidence-based treatment of women with an extremely common form of breast cancer, while delaying surgery and hospitalization during the pandemic.
These were the preliminary findings of a new study presented this week at the American Society of Breast Surgeons (ASBrS) Annual Meeting. The study also found that fewer immediate breast reconstructions were performed due to a growth in outpatient surgeries to avoid patient hospitalizations.
According to Lee Wilke, MD, Professor of Surgery from the University of Wisconsin School of Medicine and Public Health, lead researcher, the study presents an accurate snapshot of treatment changes wrought by the pandemic and was based on a representative geographic and ethnic cross section of patients entered into an ASBrS database. “Interestingly,” she says, “COVID-related breast cancer treatment changes may have provided the context for testing new protocols, with some likely to persist beyond the pandemic.”
The preliminary analysis focused on data collected from 2,476 patients entered in an embedded COVID segment in the ASBrS Mastery Program registry, which tracks the patient outcomes of ASBrS member surgeons, as well as 2,303 within the Mastery registry itself from March 1, 2020 to October 28, 2021. Data through March 15, 2021 showed similar trends and is now available.
Patient information was entered by 172 ASBrS breast surgeons nationwide and indicated whether treatment was consistent with usual protocols or modified due to COVID. Researchers found that NET was used to treat an additional 36% of patients with ER+/HER2- cancers than would have otherwise received the therapy. Patients who would have received NET without COVID ranged from 6.5% to 7.8% in the two segments of the registry. While the initial analysis reflects only data through the fall of 2020, according to Dr. Wilke, trends are likely to remain consistent throughout the study period.
She notes that many women with ER+ cancers, which generally have a good prognosis, receive anti-estrogen endocrine therapy at some point during their treatment. This therapy blocks or decreases the ability of hormones to promote the growth of certain types of cancer cells. However, most patients in the study would have received endocrine therapy following surgery if deemed appropriate—not in advance.
“During COVID, however, these drugs provided a way to start cancer treatments when hospitalization for breast cancer therapy was difficult or impossible due to operating room (OR) restrictions, with surgery then scheduled for a later date,” she says. “The rise in NET showed significant regional variation appearing earlier in the Northeast and Southeast U.S., correlating with earlier COVID infection spikes in these areas.”
Dr. Wilke also notes that treating ER+ cancers with neoadjuvant endocrine therapy was part of an initiative of the ASBrS and other well-recognized cancer societies to develop algorithm-based, tiered guidelines for alternative treatments to help hospitals avoid surgeries when possible. “Especially early during the pandemic these revised treatments were necessary because access to hospital ORs was limited or unavailable. These algorithmic-based treatment guidelines allowed us to offer high-quality evidence-based care fine-tuned for a patient’s specific cancer profile.”
“Physicians also developed a series of options for further evaluating patients with the goal of delivering the most appropriate care possible,” she notes. “For example, genomic testing on a core biopsy assessed the ability to forgo chemotherapy and thereby use NET or proceed to surgery. Patients that needed standard approaches still got them. Women with aggressive triple negative and HER2+ tumors were treated standardly with neoadjuvant chemotherapy.”
Researchers will continue to some follow patients post-pandemic to assess the effectiveness of COVID treatment strategies and the impact on their outcomes.
Dr. Wilke believes certain changes in treatment protocols may continue into the future. “Internationally, NET is used far more frequently than in the U.S. with good results,” she says. “Perhaps in the U.S., we will see a shift in protocols with an increasing comfort level of endocrine therapy as an initial treatment for appropriate cancers.”
*For full study data, which show similar trends, contact jphillips@healthflashmarketing.com.
Abstract, Official Proceedings
Analysis of the Impact of the COVID-19 Pandemic on the Multidisciplinary Management of Breast Cancer: Review from the First 8 Months of the American Society of Breast Surgeons COVID and Mastery Registries
Authors: Lee G Wilke1, Judy Boughey2, Toan T Nguyen3, Jill Dietz4, Bret Hanlon5, Qiuyu Yang5, Eric A Brown6, Kathryn A Wagner7, Pamela Strickland8
Institutions: 1University of Wisconsin School of Medicine and Public Health, Madison, WI, USA. 2Mayo Clinic, Rochester, MN, USA. 3Lakeland Regional Medical Center, Lakeland, FL, USA. 4ASBrS, Cleveland, OH, USA. 5University of Wisconsin-Madison, Madison, WI, USA. 6Comprehensive Breast Care, Troy, MI, USA. 7Texas Breast Specialists, San Antonio, TX, USA. 8UAB Medicine, Montgomery, AL, USA
Objective: The COVID-19 pandemic resulted in rapid and regionally different approaches to breast cancer care as well as prioritization based on tumor subtype. We hypothesized that the percentage of patients with estrogen positive cancer receiving neoadjuvant endocrine therapy (NET) would increase. In order to evaluate this and other changes, a COVID specific registry was developed within the American Society of Breast Surgeons (ASBrS) Mastery program. Herein we report the initial impact of the COVID-19 pandemic through analysis of the first 8 months of the COVID-19 specific as well as Mastery registries.
Methods: In March 2020, surgeons began entering patient demographic data as well as surgical and medical care (NET vs Neoadjuvant chemotherapy (NCT)) into an embedded COVID-19 specific registry. Data fields tracked whether decisions were usual for that practice, or modified due to COVID-19. Surgeons could also continue to enter data into the Mastery without COVID-19 specific information.
Results: Between 3/1 and 10/28/2020, 172 surgeons entered data on 2476 unique COVID registry and 2303 Mastery registry patients with receptor data. The majority of surgeons entered 10 to 99 patients (73%), were from urban/large urban areas (65%) and reported stopping mammographic screening during part of this time period (95%). Regional surgeon distribution was Northeast 38%, Midwest 22%, Southeast 17%, Southwest 12%, Northwest 11%. Mean patient age was 62.7, with 9.3% African American and 6.4% Hispanic.
In the COVID-19 registry initial consultation occurred infrequently via telehealth (6.8%; 170) and only 1.5% developed COVID-19 (38). The mean invasive tumor size was 2.1cm, 19% were node positive (389), and 11% (214) triple negative. Table 1 describes treatment approaches for these patients.
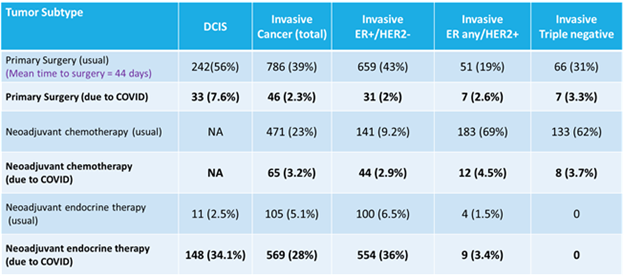
Table 1: Treatment approach in the COVID-19 registry by approximated subtype
IN ER +/HER2-disease, NET was used as a “usual” approach in 100 (6.5%) patients in the COVID-19 registry which was similar to 64 patients (7.8%) undergoing NET in the Mastery. NET was used due to COVID-19 in an additional 554 (36%) patients. In multinomial regression with surgery first/usual practice as the reference, patients were more likely to receive NET due to COVID-19 with increasing age as well as if they lived in the North or Southeast (OR 1.1, 2.2 and 1.6; p<0.05).
Genomic testing was performed on 643 patients, of which 154 (24%) had the testing done on the core due to COVID-19. Patients were less likely to have genomic testing on the core due to COVID-19 if they were older (OR 0.89; p=0.01) and more likely if they were node positive (OR 3.9; p<0.05). A change in surgical approach due to COVID-19 was reported for 269 patients (10.8%) with primary reasons noted as planned return for mastectomy (27%) or reconstruction (15%).
Conclusions: Greater than 1/3 of patients with ER positive breast cancer were treated with NET due to COVID-19. Genomic testing on cores helped guide first treatment decisions. More than 10% of patients will return for another surgery. The ASBrS COVID-19 registry represents an important platform to report management changes encountered during the initial 8 months of the pandemic and highlights both treatment changes as well as adherence to standard therapeutic approaches.