BioSig Technologies, Inc., a medical technology company commercializing a biomedical signal processing platform designed to improve signal fidelity and uncover the full range of ECG and intra-cardiac signals, has announced that it increased the patient case goal from 1000 to at least 1500 procedures by the end of 2021.
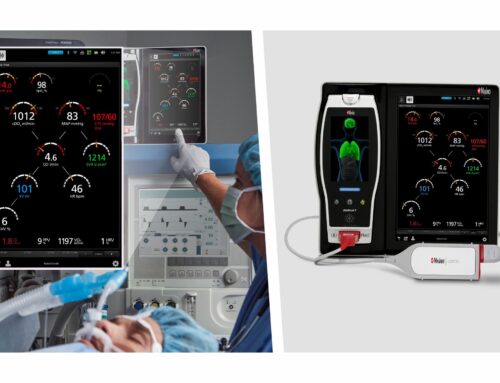
BioSig’s non-invasive computerized technology, the PURE EP™ System, aims to drive procedural efficiency and efficacy in electrophysiology. The system provides essential diagnostic signals with high clinical value in all cardiac ablations that treat irregular heartbeats or arrhythmias.
Previously the Company announced its target to complete 1000 patient cases in 2021, having delivered 425 procedures by the end of 2020. The Company is currently conducting patient cases in nine medical centers across the country. Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin, TX, the Company’s first commercial customer, continues to be the biggest user of the technology with over 300 patient cases conducted to date. Mayo Clinic Florida Campus and the University of Pennsylvania are the second and third largest patient case drivers with 130 and 112 cases1. Over 800 procedures have been conducted with the PURE EP™ System in the last 18 months.
“Patient case volume is one of the leading indications of physician utilization of our technology. We see steady procedural growth in almost all of our centers, which we believe will turn into commercial revenues. This case growth, combined with the consistently positive customer feedback, positions us well to deliver on our target of 20 installation sites by the end of 2021,” commented Kenneth L. Londoner, Chairman and CEO of BioSig Technologies, Inc.
One in 18 Americans suffers from cardiac arrhythmia. Atrial fibrillation is the most common arrhythmia type, affecting over 33 million people worldwide, including over 6 million in the U.S. The number of people suffering from atrial fibrillation is expected to reach 8-12 million by 20502. According to the Centers for Disease Control and Prevention (CDC), atrial fibrillation causes more than 750,000 hospitalizations in the U.S. each year, resulting in approximately $6 billion in healthcare spending annually3.
About BioSig Technologies
BioSig Technologies is a medical technology company commercializing a proprietary biomedical signal processing platform designed to improve signal fidelity and uncover the full range of ECG and intra-cardiac signals (www.biosig.com).
The Company’s first product, the PURE EP ™ System is a computerized system intended for acquiring, digitizing, amplifying, filtering, measuring and calculating, displaying, recording and storing of electrocardiographic and intracardiac signals for patients undergoing electrophysiology (EP) procedures in an EP laboratory.