Increasingly Practice Guidelines Recommend Image Guidance for Spinal Needle Placement Procedures
CHARLOTTESVILLE, VA., February 19, 2020 — RIVANNA® today announced that sales of its Accuro ultrasound-based spinal navigation system more than doubled in 2019 compared to the previous year, citing the impact of a growing number of new healthcare practice guidelines recommending ultrasound guidance for spinal needle placement procedures. Accuro automates ultrasound image detection of spinal landmarks, including the epidural space, thereby eliminating the modality’s steep learning curve, and guides users to the optimal spinal location and depth for needle placement. Unlike conventional ultrasound systems, it enables even novice users to take advantage of precise image guidance rather than blind palpation for needle placement, helping to avoid multiple insertions, redirects and potential related complications.
“Manual spinal palpation has been used for decades to estimate the spinal midline and appropriate intervertebral space for needle placement in anesthesia delivery or intrathecal access procedures,” says Will Mauldin, PhD, co-founder and CEO of RIVANNA. “However, healthcare has been moving toward an image-driven standard of care for spinal needle placement procedures and updated practice guidelines increasingly recommend ultrasound-based techniques.”
He notes that a Society of Hospital Medicine Position Statement published online in The Journal Hospital of Medicine recommends ultrasound guidance for optimal lumbar puncture site selection to minimize complications. Recently the American Association of Nurse Anesthetists revised their Analgesia and Anesthesia for the Obstetric Practice Guidelines to cite neuraxial ultrasound as a useful adjunct in patients with difficult-to-palpate anatomic landmarks. In addition, The National Institute for Health and Clinical Excellence (NICE) issued full guidance to the National Health Service throughout Great Britain highlighting the value of ultrasound imaging in identifying the epidural space.
“Clearly, the value of ultrasound for these procedures is gaining acceptance,” says Mauldin. “However, with conventional ultrasound devices, use is limited from a practical standpoint. Accuro was specifically developed to overcome the shortcomings of ultrasound use for spinal needle placement applications. We believe all this is an important factor in the growth of our sales.”
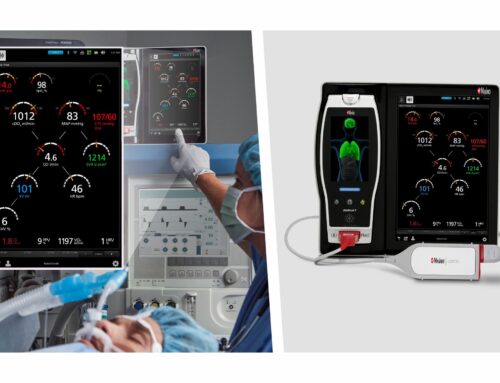
Relying on automated SpineNav3D™ technology, Accuro enables users with even minimal ultrasound experience to navigate the lumbar spine and identify relevant landmarks, which are used to compute, visualize and pinpoint the optimal needle insertion point.
Moreover, Accuro BoneEnhance® technology compensates for ultrasound’s inherent bony structure distortion with a fivefold increase in bone-to-tissue contrast for greater image clarity and accuracy. The device also features an innovative ultrasound transducer type known to limit certain bone imaging artifacts present in conventional ultrasound systems.
The device’s flexible, handheld form factor enables easy and convenient operation and supports conventional workflow. The result is a device that easily and conveniently brings the power of image-guided spinal needle placement to all healthcare providers.
A study found that the SpineNav3D technology had accuracy exceeding 94% in automatically locating the epidural space compared to expert radiology reviewers (Tiouririne et al., 2017). A growing number of clinical trials are demonstrating significant product benefits for users, whatever their ultrasound experience level. This includes a 48% reduction in needle redirects, a 57% reduction in needle insertion time and improved patient satisfaction reaching 95% (M. Tiouririne et al., 2017; Singla et al., 2019; D. Ghisi et al., 2019).
Currently Accuro is sold in more than 20 counties and will enter the market in Mexico, South Korea and Australia and New Zealand in 2020. Accuro’s 87% trial-to-purchase conversion attests the product’s value to its customers.
“I believe we are at a watershed moment,” adds Mauldin. “We are witnessing a major migration away from blind palpation-based needle placement in favor of a modern, safer and more efficient image-guided standard for spinal needle placement procedures. That is good news for patients and providers alike.”
About RIVANNA and Accuro
Accuro® by RIVANNA® is the world’s first spinal navigation device designed to improve the safety, speed, and efficiency of epidural and spinal anesthesia. The revolutionary platform features BoneEnhance®, which optimizes ultrasound for the visualization of bony versus soft tissue anatomy, and SpineNav3D™, which automates measurements of the spinal midline, epidural depth and trajectory. Accuro was engineered and commercialized by RIVANNA, an innovative medical device company headquartered in Charlottesville, VA. For anesthesia providers, certainty can be effortless with Accuro. For more information, visit rivannamedical.com.
CONTACT:
Jeanne-Marie Phillips
HealthFlash Marketing
203-977-3333