By Eric Mayes, CEO of Endomag |
If you, or a loved one, have ever experienced an early-stage breast cancer diagnosis, you’ll probably be familiar with the process of axillary surgery to ‘stage’ the cancer and understand the extent of any possible spread. This is performed using a tracer, which is injected into the breast and travels to the lymph nodes in the underarm. Using a handheld probe, the surgeons then locate where they need to operate.
For many decades, the tracers used have been radioactive medicines – a highly effective, but equally high burden solution due to storage logistics and supply issues – combined with blue dye. However, many breast care teams are now phasing this method out in favour of a magnetic alternative, which could be about to revolutionize the way we stage breast cancer.
The challenges of using radioactive tracers
Although radioactive tracers are highly effective and are commonly used to stage cancers such as breast cancer, they have their drawbacks.
The most common radioactive isotope used for breast cancer staging is technetium-99m. It carries a short half-life of just six hours, which means that every six hours, the radioactive signal surgeons use to locate lymph nodes halves. As a result, the supply of radioactive isotopes is quite fragile, and they must be prepared locally and used quickly. In some cases, hospitals have been forced to stage patients with substandard alternatives when the supply chain hasn’t delivered on time.
Once radioactive tracers arrive, hospitals need to ensure they have the facilities in place to use them. They require dedicated nuclear medicine departments where they can be prepared and then injected due to strict regulatory controls. For many smaller clinics access to these facilities is not easy to provide, leaving them unable to stage cancer.
The COVID-19 pandemic placed a spotlight on this issue, when many cancer surgeries were moved to ‘clean’ COVID-19 free sites to continue. These independent hospitals and smaller day-case units often lacked the necessary nuclear medicine facilities to store, prepare and inject radioactive tracers, meaning patients were not always able to receive the best quality of care possible.
Lastly, radioactive tracers are often used in combination with a blue dye, which provides surgeons with a visual reference point. In a small, but not to be overlooked number of cases, studies have shown that some patients have experienced severe allergic reactions to the dye. For these patients, there is a need for alternative solutions, to stage and assess their cancer.
The alternative
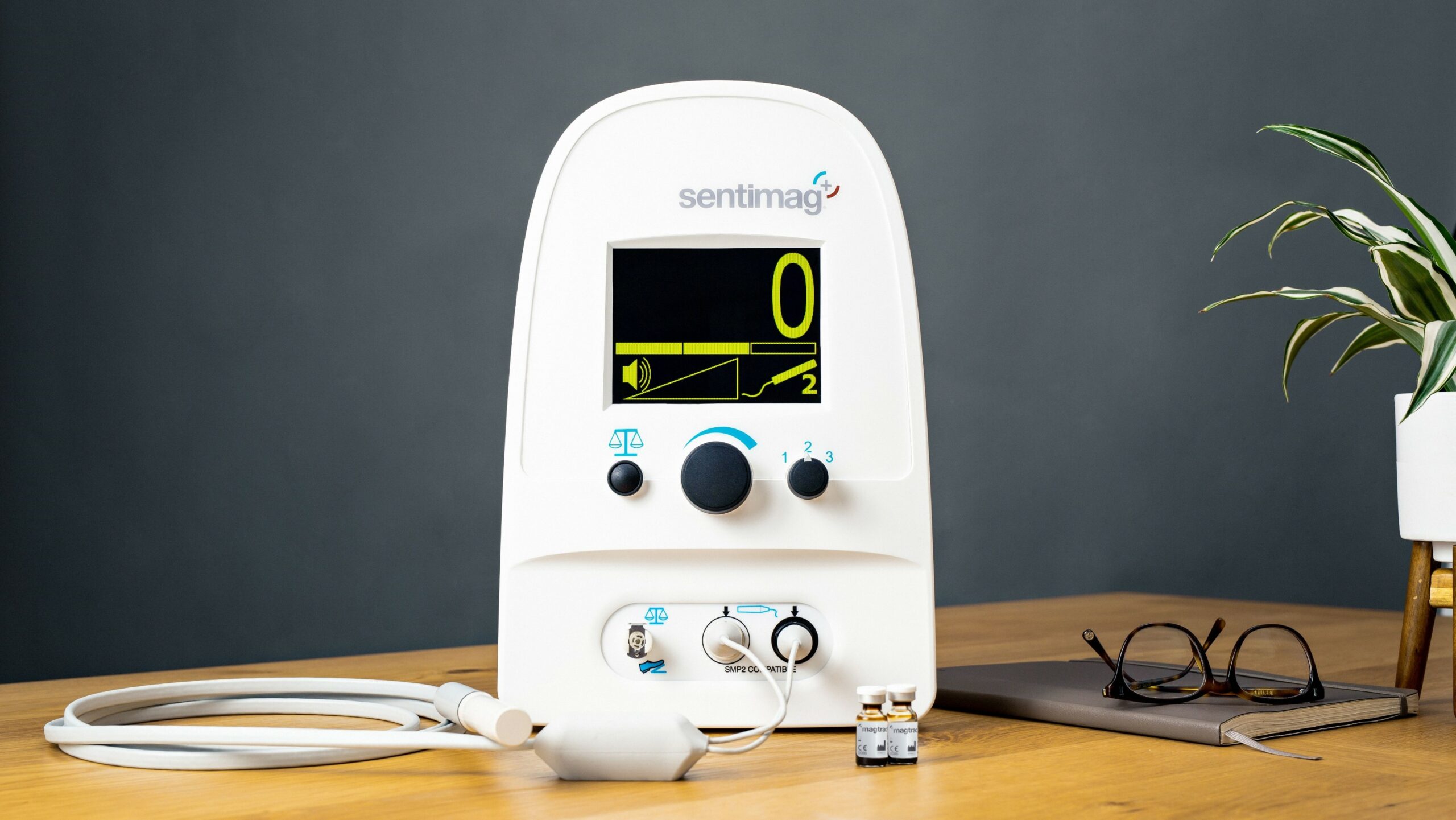
With ongoing innovation in this area of breast cancer, new technologies are emerging. One of these is highly optimized magnetic technology, designed to remove the need for radioactivity when staging breast cancer. So how do these magnetic tracers work?
Instead of a radioactive signal, these tracers emit a magnetic signal when activated by a handheld probe that also guides doctors to the site of the suspected cancerous tissue. To mark the lymph nodes, tiny magnetic particles suspended in liquid are injected into the breast and quickly follow the same path a spreading cancer cell would take.
The Magtrace lymphatic tracer can be injected any time between 20 minutes and 30 days ahead of the staging procedure, known as a ‘sentinel lymph node biopsy’, reducing the patients’ time spent in hospital and enabling more efficient use of hospital resources for the care team.
The appeal of magnetic tracers
The introduction of magnetic tracers has seen them grow to become a popular choice for cancer staging across the world. It’s easy to see why.
First, magnetic tracers allow breast cancer staging to take place anywhere, whether the clinic has access to nuclear facilities or not. Magnetic tracers could be used in the largest hospital in the country or a small remote centre, serving their rural community.
As the signal does not decay, magnetic tracers can be easily stored and shipped, allowing them to be used anywhere around the world. This means patients everywhere can now access the best care, something that hasn’t been possible up until now.
An additional benefit of the magnetic solution is that the injection time window of the tracer also makes new surgical techniques possible. For example, in the US magnetic tracers can enable a pioneering new technique known as a ‘delayed’ sentinel lymph node biopsy. This new surgery is suitable for women diagnosed with high-risk ductal carcinoma in-situ (DCIS) who are undergoing a mastectomy.
Until now, when women with DCIS opted for a mastectomy to remove their cancer, doctors would also remove lymph nodes in the underarm at the same time, as a precautionary measure in case the DCIS has become invasive. Yet, studies have shown that up to 80% of women diagnosed with DCIS do not have invasive cancer, rendering this additional surgery unnecessary for them.
Using magnetic tracers, physicians in the US are now able to mark the lymph nodes at the time of performing the mastectomy, only removing the DCIS initially. They are then able to send that tissue to pathology for further analysis to find out if the cancer is invasive, before deciding if underarm surgery is actually needed. For many women, this is now eliminating the need for underarm surgery altogether.
Is the future magnetic?
While nuclear medicine will continue to play an important role in the diagnosis and treatment of cancer, many believe that magnetic tracers offer significant advantages when it comes to cancer staging. It is vital that patients can access the best diagnostic and treatment options available at every stage of the cancer care pathway.
With over 600 hospitals in 45 countries having already adopted magnetic tracer technology, the revolution has started – and that can only be a good thing.
 About the Author
About the Author
Dr Eric Mayes is CEO of Endomag and has over 20 years’ experience in developing and leading materials technology companies spanning data storage, display technology, printed electronics and medical devices. Eric has led Endomag through its rapid growth since marketing its first device for staging breast cancer in late 2012. The company has now helped over 130,000 patients receive a higher standard of care in over 550 hospitals across 43 countries.
Eric holds a BSc in Physics from Arkansas State University, a PhD in Chemistry from the University of Bath and is a Fellow of the Royal Society of Chemistry.
*Magtrace® is currently indicated for use in patients undergoing mastectomy only in the US.