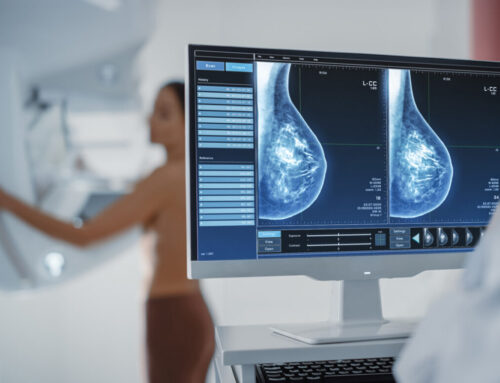
Zebra Medical Vision, the deep-learning medical imaging analytics company, announces today its sixth FDA 510(k) clearance for its mammography solution, HealthMammo, which has already received a CE mark. Zebra Medical’s algorithm empowers breast radiologists by prioritizing and identifying suspicious mammograms, providing a safety net for radiologists. The suspicious mammograms are identified faster and read earlier than the current “first-in first-out” standard of care.
This is Zebra Medical Vision’s first solution for Oncology to receive FDA clearance, as part of the company’s AI1 “All-In-One” bundle. Zebra Medical is also the first startup to receive FDA clearances for AI based technology across three imaging modalities – CT, x-ray and mammography, providing coverage for roughly 80% of the total imaging volumes.
Breast cancer is the most frequently diagnosed cancer among women, impacting 2.1 million women worldwide each year and causing the greatest number of cancer-related deaths among women. It is estimated that 627,000 women died from breast cancer in 2018, approximately 15 percent of all cancer-related female deaths. While breast cancer rates are higher among women in more developed regions, rates are increasing in nearly every region globally.
Zebra Medical’s HealthMammo solution is an automatic AI tool that indicates “suspicious” or “not suspicious” for every 2D mammography performed. The mammograms are automatically sent to Zebra Medical’s imaging analytics platform, where they are processed and analyzed for suspected breast lesions. The HealthMammo product then returns its result to the radiologist, either by signaling within the worklist or by notifying the user in a dedicated application.
The algorithm can be used in single reader paradigms, supporting triage and workflow improvement (as cleared by the FDA) or in double reading paradigms, already available outside of the US, supporting the second radiologists and reducing their workload.
Supporting early detection protocols, Zebra Medical Vision’s HealthMammo product helps health providers minimize the COVID-19-induced care gaps with an automated AI tool that can identify faster patients with suspicious lesions. According to the United States Food & Drug Administration (FDA) Mammography Quality Standards program (MQSA), approximately 40 million mammograms are performed in the U.S. every year. With the outbreak of coronavirus, annual mammography tests during lockdown were postponed or canceled. As a result, providers are dealing with substantial growing backlogs, as 100,000 screens are added every day in the US alone, in addition to a 94% drop in mammography imaging volumes during lockdown. Patients are experiencing increasing anxiety waiting to be tested and run the risk of missing the early detection component of annual screening which could result in an undetected cancer that keeps growing.
“As restrictions are lifted from the COVID-19 crisis, the backlog of mammograms has increased,” says Dr. Michael Fishman, Breast Imaging Section Chief at Boston Medical Center (BMC), Massachusetts. “Zebra Medical Vision’s HealthMammo may help radiologists deal with the screening management strategy of the post COVID backlog and triaging.”
“Our work is twofold: supporting the medical team’s overload and ensuring the well-being of patients, by supporting early detection and reducing the anxiety surrounding uncertainty,” says Ohad Arazi, CEO of Zebra Medical Vision. “The fact that during initial testings we were able to identify 2 cases that were missed, and to have these women be recalled and diagnosed with cancer, shows the vast impact and potential contribution of AI in Oncology. With this fully commercial and regulated product, we aim to provide even more value and help patients and providers navigate the new COVID effected reality we are all facing. We’re proud of the achievements we’ve made in the past few months, providing U.S. healthcare with a growing portfolio of automatic solutions to enhance patient care, especially during these times.”