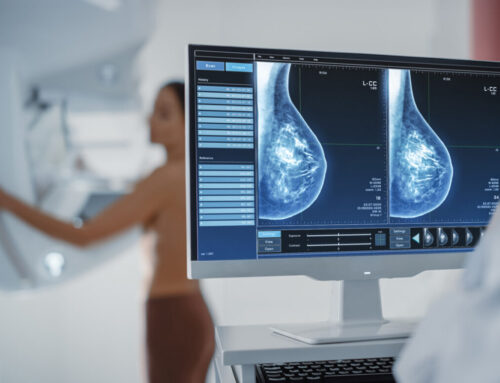
Endomag, a global medical technology company, has received a positive Medtech Innovation Briefing (MIB) from the National Institute for Health and Care Excellence (NICE) for their Magtrace lymphatic tracer’s ability to locate sentinel lymph nodes. Magtrace becomes the first and only tracer to receive this endorsement. According to Endomag, it allows any patient or hospital to access the highest standard in breast cancer staging, without the need for nuclear facilities or radioisotopes.
Endomag, a global medical technology company, has received a positive Medtech Innovation Briefing (MIB) from the National Institute for Health and Care Excellence (NICE) for their Magtrace lymphatic tracer’s ability to locate sentinel lymph nodes. Magtrace becomes the first and only tracer to receive this endorsement. According to Endomag, it allows any patient or hospital to access the highest standard in breast cancer staging, without the need for nuclear facilities or radioisotopes.
NICE’s positive review is the UK’s national mark of excellence, informing local National Health Service decision-makers how to provide better patient care. It was published as part of a Medtech Innovation Briefing commissioned by NHS England in support of the NHS 5-Year Forward View, to accelerate innovation in new treatments and diagnostics.
Sentinel lymph nodes are lymph nodes adjacent to a cancerous tumor and are examined by doctors to determine if the cancer has spread to other parts of the body. Lymphatic tracers are used to mark these nodes for biopsy. Traditionally, a radioactive isotope, technetium-99m, and blue dye is used as a lymphatic tracer. Magtrace instead uses magnetic particles and signals to mark the sentinel lymph nodes. It can be injected any time between 20 minutes and 30 days ahead of a sentinel lymph node biopsy, reducing the patients’ time in hospital and enabling the more efficient use of NHS resources.
Since the UK left the European Union in January 2020, it also left Euratom, a commissioning body which governs the use and transport of radioactive material like technetium-99m. Subsequent delays have resulted in some NHS hospitals staging breast cancer with just blue dye, associated with a high false negative rate of 13%, as well as a risk of allergic reaction, or anaphylaxis. [1], [2]
“A sentinel lymph node biopsy is the standard of care for staging early breast cancer and is vital in helping us understand whether cancer has spread,” explains Kate Williams, Consultant Oncoplastic Breast Surgeon at North Manchester General Hospital, Manchester Foundation Trust, and one of the experts that contributed to Magtrace’s NICE’s briefing. “It’s concerning to hear that women with breast cancer are having this procedure with substandard techniques when there are non-radioactive alternatives like Magtrace, ready and available.”
Unlike technetium-99m, the Magtrace manufactured in the UK and requires no nuclear facilities to be stored or used. It is also well-tolerated and carries no risk of allergic reactions. [3]
“Breast cancer is better understood than any other cancer today. And yet, the need for innovation has never been greater, as so many patients have been missed during the pandemic. We feel more determined than ever to make a difference after this NICE positive review, which confirms that innovative UK technologies like the Magtrace marker can play a role to help the health system continue staging breast cancer,” said Eric Mayes, CEO, Endomag.
References
[1] NICE. Magtrace and Sentimag for locating sentinel lymph nodes. Available at: https://www.nice.org.uk/advice/mib263 Last Accessed, August 2021.
[2] Li J, et al. Sentinel lymph node biopsy mapped with methylene blue dye alone in patients with breast cancer: A systematic review and meta-analysis. PLoS One. 2018;13(9):e0204364. Published 2018 Sep 20.
[3] Zada A, et al. Meta-analysis of sentinel lymph node biopsy in breast cancer using the magnetic technique. Br J Surg. 2016 Oct;103(11):1409-19.
By Ian Schnepf, Content Writer for HealthTech HotSpot