Three-year Results of the ICE3 Trials
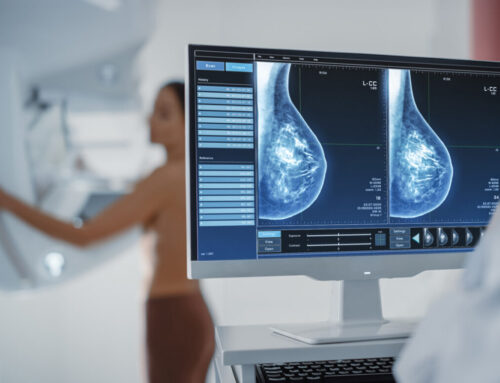
Non-surgical breast cancer cryoablation, which destroys tumor cells by exposing them to sub-freezing temperatures, is proving to be an effective alternative to surgery for small breast cancers, with low-risk features, in women over 60 years, based on the early three-year results of an ongoing IRB-approved clinical trial. The findings of the multi-center ICE3 trial—the first ever cryoablation trial that does not involve follow-up surgery—was presented at the American Society of Breast Surgeons (ASBrS) annual meeting this week.
“Cryoablation potentially represents a dramatic improvement in care for appropriate low-risk patients, and at three years post-treatment, the ICE3 trial results are extremely positive,” says Richard Fine, MD, West Cancer Center & Research Institute, lead researcher. “The non-invasive procedure is fast, painless and can be delivered under local anesthesia in a doctor’s office. Recovery time is minimal and cosmetic outcomes are excellent with little loss of breast tissue and no scarring. Now, this trial is underscoring the efficacy and safety of the procedure for this patient group.”
Dr. Fine notes that this clinical trial builds on others demonstrating that cryoablation of small, low-grade tumors is effective. The technique also is used routinely as therapy for other types of cancers.
Researchers studied 194 patients 60 years of age or older with unifocal invasive ductal cancers measuring 1.5 cm or less. Tumors were all low-grade, HR+, HER2-, consistent with a low-risk form of the disease. Women were treated with a cryoablation freeze-thaw-freeze cycle for 20 to 40 minutes. Treatment was delivered through a needle-like nitrogen-chilled probe inserted through the skin directly into the tumor. Freezing temperatures targeted a carefully controlled area, turning the tumor into an ice ball to destroy the diseased cells. No surgical incision and related tissue damage and scarring were involved.
Along with cryoablation, at the discretion of their treating physician, 27 patients received or are receiving adjuvant radiation, 148 with endocrine therapy and one with chemotherapy. All patients were followed at six month intervals with same breast recurrence at five years after cryoablation as the primary trial outcome.
At a mean of 34.83 months from treatment, only 2.06% (4 patients) recurred. Ninety-five percent of patients and 98% of treating physicians reported satisfaction with the cosmetic results. No significant device-related adverse events were reported.
One patient had breast cancer-related positive sentinel lymph nodes at biopsy. That patient remains cancer-free at 60 months follow up.
“Increasingly, precision medicine is helping physicians characterize breast cancer with tools like genomic profiling and hormone receptor status,” Dr. Fine says. “We also are picking up cancers at an earlier stage than ever before. In recent years, healthcare has been deescalating breast cancer treatment, realizing that for some tumors, less aggressive therapies can be as effective and deliver greater patient satisfaction at a lower cost than traditional interventions. In keeping with that trend, cryoablation is a promising, high value treatment for certain forms of less aggressive cancers.”